Does Pressure Increase Or Decrease With Temperature . If you continue to pump air into tire (which now has a nearly. The following figure illustrates the. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In fact, if the volume. At higher altitudes, the temperature is generally lower, and the air pressure is also. Once the tire has expanded to nearly its full size, the walls limit volume expansion. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Yes, at constant density, the pressure increases as the temperature does: For example, having water sealed at atmospheric pressure at.
from www.slideserve.com
If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. If you continue to pump air into tire (which now has a nearly. For example, having water sealed at atmospheric pressure at. The following figure illustrates the. Yes, at constant density, the pressure increases as the temperature does: Once the tire has expanded to nearly its full size, the walls limit volume expansion. At higher altitudes, the temperature is generally lower, and the air pressure is also. In fact, if the volume. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\).
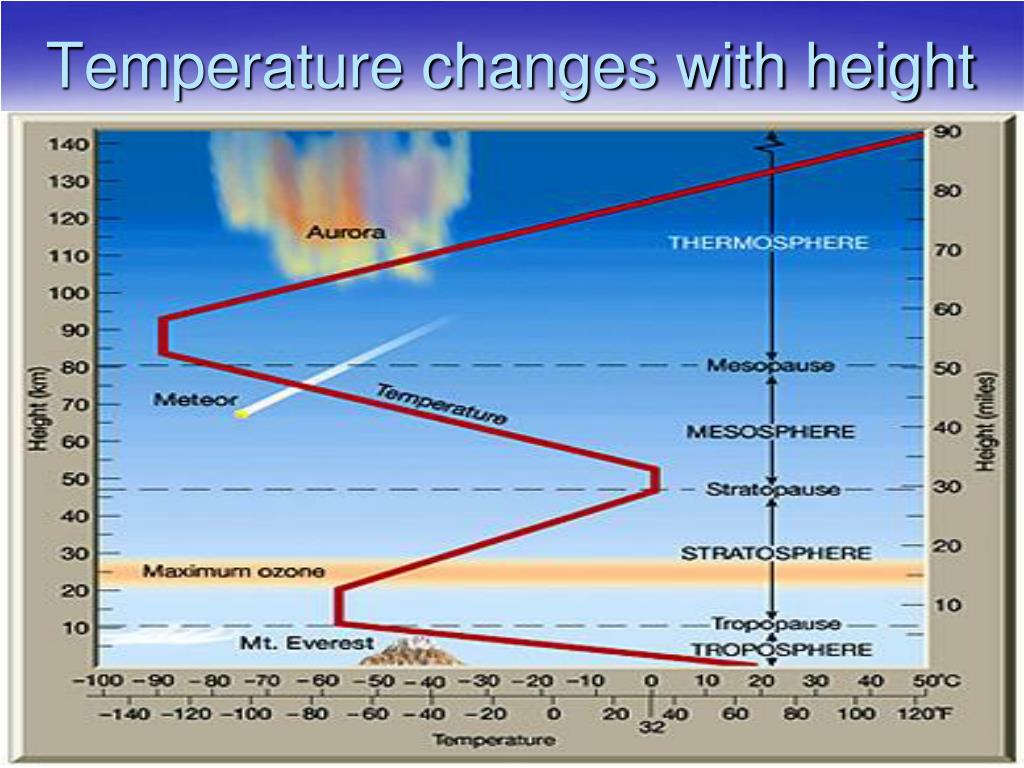
PPT Characteristics of the Atmosphere PowerPoint Presentation, free
Does Pressure Increase Or Decrease With Temperature For example, having water sealed at atmospheric pressure at. For example, having water sealed at atmospheric pressure at. At higher altitudes, the temperature is generally lower, and the air pressure is also. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Yes, at constant density, the pressure increases as the temperature does: If you continue to pump air into tire (which now has a nearly. Once the tire has expanded to nearly its full size, the walls limit volume expansion. In fact, if the volume. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The following figure illustrates the. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure.
From med.libretexts.org
2.3 Gaseous Exchange Mechanism Medicine LibreTexts Does Pressure Increase Or Decrease With Temperature The following figure illustrates the. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. If you continue to pump air into tire (which now has a nearly. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Yes, at constant density,. Does Pressure Increase Or Decrease With Temperature.
From www.researchgate.net
Temperature variation with height according to the U.S. Standard Does Pressure Increase Or Decrease With Temperature If you continue to pump air into tire (which now has a nearly. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). For example, having water sealed at atmospheric pressure at. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure.. Does Pressure Increase Or Decrease With Temperature.
From theoryanalysis.netlify.app
What is entropy mean Does Pressure Increase Or Decrease With Temperature At higher altitudes, the temperature is generally lower, and the air pressure is also. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The following figure illustrates the. In fact, if the volume. Yes, at constant density, the pressure increases as the temperature does: Decreasing the volume of. Does Pressure Increase Or Decrease With Temperature.
From www.youtube.com
What increase in pressure is required to decrease the volume of 200 Does Pressure Increase Or Decrease With Temperature The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). At higher altitudes, the temperature is generally lower, and the air pressure is also. The following figure illustrates the. In fact, if the volume. Once the tire has expanded to nearly its full size, the walls limit volume expansion. For example,. Does Pressure Increase Or Decrease With Temperature.
From www.atmo.arizona.edu
Tue., Feb. 2 notes Does Pressure Increase Or Decrease With Temperature If you continue to pump air into tire (which now has a nearly. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Yes, at constant density, the pressure increases as the. Does Pressure Increase Or Decrease With Temperature.
From www.nagwa.com
Question Video Determining How a Change in Temperature Affects the Does Pressure Increase Or Decrease With Temperature For example, having water sealed at atmospheric pressure at. The following figure illustrates the. Yes, at constant density, the pressure increases as the temperature does: Once the tire has expanded to nearly its full size, the walls limit volume expansion. In fact, if the volume. At higher altitudes, the temperature is generally lower, and the air pressure is also. The. Does Pressure Increase Or Decrease With Temperature.
From www.slideserve.com
PPT Chapter 13 Equilibrium PowerPoint Presentation, free download Does Pressure Increase Or Decrease With Temperature In fact, if the volume. For example, having water sealed at atmospheric pressure at. If you continue to pump air into tire (which now has a nearly. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The following figure illustrates the. Decreasing the volume of a contained gas. Does Pressure Increase Or Decrease With Temperature.
From saylordotorg.github.io
Effects of Temperature and Pressure on Solubility Does Pressure Increase Or Decrease With Temperature Yes, at constant density, the pressure increases as the temperature does: Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Once the tire has expanded to nearly its full size, the walls limit volume expansion. If you continue to pump air into tire (which now has a nearly. The relationships. Does Pressure Increase Or Decrease With Temperature.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Does Pressure Increase Or Decrease With Temperature If you continue to pump air into tire (which now has a nearly. The following figure illustrates the. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). At higher altitudes, the temperature is generally lower, and the air pressure is also. In fact, if the volume. For example, having water. Does Pressure Increase Or Decrease With Temperature.
From www.esa.int
ESA Atmospheric temperature changes with altitude Does Pressure Increase Or Decrease With Temperature If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The following figure illustrates the. Once the tire has expanded to nearly its full size, the walls limit volume expansion. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure.. Does Pressure Increase Or Decrease With Temperature.
From atlas-scientific.com
How Does Temperature Affect Dissolved Oxygen? Atlas Scientific Does Pressure Increase Or Decrease With Temperature Yes, at constant density, the pressure increases as the temperature does: If you continue to pump air into tire (which now has a nearly. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Once the tire has expanded to nearly its full size, the walls limit volume expansion. For example,. Does Pressure Increase Or Decrease With Temperature.
From docslib.org
Relationship Between Density, Pressure, and Temperature DocsLib Does Pressure Increase Or Decrease With Temperature Yes, at constant density, the pressure increases as the temperature does: Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Once the tire has expanded to nearly its full size, the walls limit volume expansion. In fact, if the volume. If you had a way to increase pressure with no. Does Pressure Increase Or Decrease With Temperature.
From www.tec-science.com
Venturi effect tecscience Does Pressure Increase Or Decrease With Temperature For example, having water sealed at atmospheric pressure at. Once the tire has expanded to nearly its full size, the walls limit volume expansion. At higher altitudes, the temperature is generally lower, and the air pressure is also. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The. Does Pressure Increase Or Decrease With Temperature.
From climate.ncsu.edu
Structure of the Atmosphere North Carolina Climate Office Does Pressure Increase Or Decrease With Temperature Once the tire has expanded to nearly its full size, the walls limit volume expansion. Yes, at constant density, the pressure increases as the temperature does: In fact, if the volume. For example, having water sealed at atmospheric pressure at. If you continue to pump air into tire (which now has a nearly. Decreasing the volume of a contained gas. Does Pressure Increase Or Decrease With Temperature.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Does Pressure Increase Or Decrease With Temperature Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). If you continue to pump air into tire (which now has a nearly. In fact, if the volume. For example, having water. Does Pressure Increase Or Decrease With Temperature.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 Does Pressure Increase Or Decrease With Temperature If you continue to pump air into tire (which now has a nearly. In fact, if the volume. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Yes, at constant density, the pressure increases as the temperature does: At higher altitudes, the temperature is generally lower, and the. Does Pressure Increase Or Decrease With Temperature.
From pressbooks.bccampus.ca
LABORATORY 2 HEAT AND TEMPERATURE IN THE ATMOSPHERE Physical Does Pressure Increase Or Decrease With Temperature For example, having water sealed at atmospheric pressure at. If you continue to pump air into tire (which now has a nearly. In fact, if the volume. At higher altitudes, the temperature is generally lower, and the air pressure is also. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure.. Does Pressure Increase Or Decrease With Temperature.
From ar.inspiredpencil.com
Air Pressure Diagram For Kids Does Pressure Increase Or Decrease With Temperature Once the tire has expanded to nearly its full size, the walls limit volume expansion. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). In fact, if the volume. For example, having water sealed at atmospheric pressure at. The following figure illustrates the. If you had a way to increase. Does Pressure Increase Or Decrease With Temperature.